Advanced Wound Treatment with mVASC
Learn more about how mVASC can enhance healing

MVT has developed an off-the-shelf, ready to use product that capitalizes on the structural properties of microvascular tissue. Each mVASC package contains a 19.5mm diameter disk of human microvascular extracellular matrix embedded in a cryopreservative and crystalloid. The microvascular tissue is derived from human cadaveric subcutaneous tissue that has been aseptically processed and lyophilized. mVASC enhances the healing potential by providing a foundation for tissue repair and may increase blood flow. mVASC is terminally sterilized and room temperature stable for 5 years. Please see mVASC product instructions for use for complete product information.
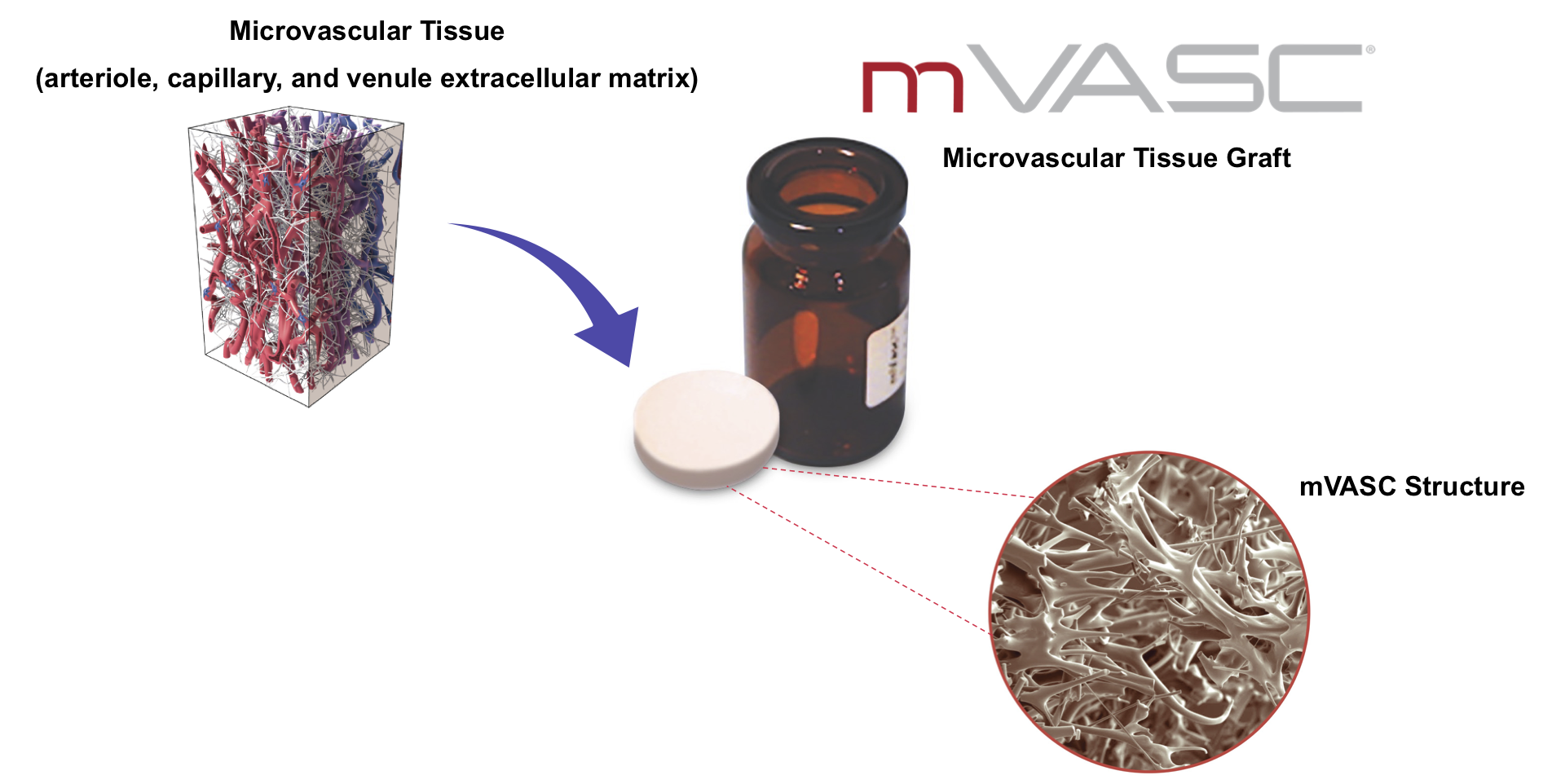
mVASC is marketed in accordance with FDA HCT/P regulations, and is restricted to homologous use for the repair of damaged microvascular tissues. It can be used wherever microvascular deficiencies exist. For example, mVASC can be added to an ischemic wound to improve healing and blood flow.
The composition and function of mVASC has been extensively studied in recognized preclinical models: including mouse pressure ulcer and hindlimb ischemia studies, which demonstrated that mVASC improved blood flow, resulting in quality tissue healing.
mVASC demonstrates significantly greater re-perfusion and vascular reconstruction in a recognized mouse angiogenesis model.
Dobke M, et al. Microvascular tissue as a platform technology to modify the local environment and influence the healing cascade. Regn Med, 15(2):1313(2020)
mVASC significantly increased the rate of wound healing in a recognized mouse pressure ulcer model, demonstrating its potential as an advanced treatment for restoring normal tissue function in ischemic wounds.
Gimble J et al. A Novel, Sterilized Microvascular Tissue Product Improves Healing in a Murine Pressure Ulcer Model. Plastic and Reconstructive Surgery – Global Open,6(11):e2010 (2018).
mVASC demonstrates significantly more newly formed blood vessels and more mature (large diameter) vessels in a recognized angiogenesis model.
Dobke M, et al. Microvascular tissue as a platform technology to modify the local environment and influence the healing cascade. Regn Med, 15(2):1313(2020)
MVT’s peer-reviewed HIFLO trail (HEALING IN DIABETIC FOOT ULCERS WITH MICROVASCULAR TISSUE), represents a new bar for evaluating advanced wound care products. The HIFLO Trial is a 100-patient Level 1 multi-center prospective randomized controlled trial of allogeneic microvascular tissue (mVASC) in nonhealing neuropathic diabetic foot ulcers. Using four predefined criteria and an independent blinded panel of three adjudicators to determine closure, the rigorous HIFLO Trial included a real-world patient population of Wagner 1 and 2 DFUs (uncommon in a large RCT) and is the first RCT to include sub studies involving perfusion and peripheral neuropathy.
mVASC is unique among advanced wound therapies, as it represents a ‘biological matching’ of the solution to a disease state. The HIFLO Trial represents the first demonstration of using microvascular elements to treat microvascular dysfunction in a randomized study. By leveraging intrinsic healing mechanism, mVASC achieved superior wound closure and quality of healing, including a marked improvement in wound regional blood flow and significant increases in local and regional sensation.
Overall, mVASC increased the odds of healing by 9 times over standard of care treatment. The higher percentage of closed ulcers and faster time to healing with mVASC may mitigate some of the risk factors associated with DFU complications, such as infection, recurrence, and amputation. The improvements in blood flow and neuropathy may also mitigate these risk factors, though additional studies should be performed for confirmation.
with mVASC
vs. 67% Decrease with Standard of Care
with mVASC
Greater Odds of Healing with mVASC
vs. Standard of Care
Nonhealing diabetic foot ulcers (DFUs) are stalled wounds with compromised repair capabilities. Less than one quarter of these ulcers will heal with standard wound care after 12 weeks. ¹

The large clinical and economic burden of nonhealing DFUs continues to escalate, and includes multiple admissions, treatment for infection, neuropathy, and amputations. Up to 34% of the 34 million diabetics in the US will develop a DFU during their lifetime. ² ³ Despite use of advanced therapeutics, the 5-year mortality after amputation from a diabetic foot ulcer is over 45%. 4
In addition to addressing the compromised vasculature contributing to a nonhealing DFU, microvascular tissue also has the potential to impact the loss of sensation common in diabetes. Leaky capillary walls in diabetic tissue decrease blood flow to the surrounding microvascular tissues, resulting in structural changes that also damage the nerves, and ultimately leading to neuropathy associated with the occurrence and recurrence of DFUS. 5,6
A functioning microvasculature is crucial for closing nonhealing wounds and restoring an environment that translates into well-perfused, sensate, high quality tissue.
References:
2Armstrong DG, et al. N Engl J Med. 376:24 (June 2017). (up to 34% of 34M)
3National Diabetes Statistics Report, 2020. (up to 34% of 34M)
4Armstrong DG, et al. J Foot Ankle Res. 13:16 (2020). (5y mortality <45%)
5Thrainsdottir S, et al. Diabetes. 52:2615 (2003). (vascular changes lead to neuropathy)
6Lavery L, et al. Arch. Intern. Med. 158:157 (1998). (neuropathy predictor of DFU)
7Yuan, S et al. Regulation of Endothelial Barrier Function. Chapter 2: Structure and Function of Exchange Microvessels. Morgan & Claypool Life Sciences. 2010. San Rafael, CA
8Xu, J et al. Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta. 2014;1842:2106–2119
Learn more about how mVASC can enhance healing


©Copyright 2023 MicroVascular Tissues, Inc.